What is Passivation of Lithium Battery?
Cell passivation is an important characteristic of lithium battery that can be very difficult to understand for many batteries-users. This section discusses this phenomenon, and it gives suggestions on how to counteract the effects of passivation when using lithium batteries.
Passivation is a very thin, high resistant, self-assembled layer formed on the surface of the lithium anode. It is formed as a result of a chemical reaction between the battery electrolyte and the lithium anode. Without the passivation layer, this type of lithium battery would not exist because the lithium would discharge and degrade quite rapidly. An advantage of the passivation layer is it allows the battery to have a very low self discharge rate and extremely long shelf life.
The most obvious affect of the passivation layer is voltage delay. Voltage delay will occur when a load is placed on the cell as illustrated in the following drawing:
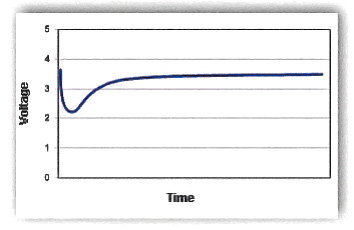
After a load is applied to a cell, the high resistance of the passivation layer causes the voltage of the cell to drop rapidly. The discharge reaction slowly removes the passivation layer thereby lowering the internal resistance of the cell. This in turn causes the voltage of the cell to reach a peak value which should remain steady if other discharge conditions do not change. If the load increases after the cell s voltage stabilizes, then it may drop again until the passivation layer is fully removed.
Once the load is removed or lowered, the passivation layer will reform, and voltage delay may be a factor when subsequent loads are applied.

